COVID-19 RAPID TESTS
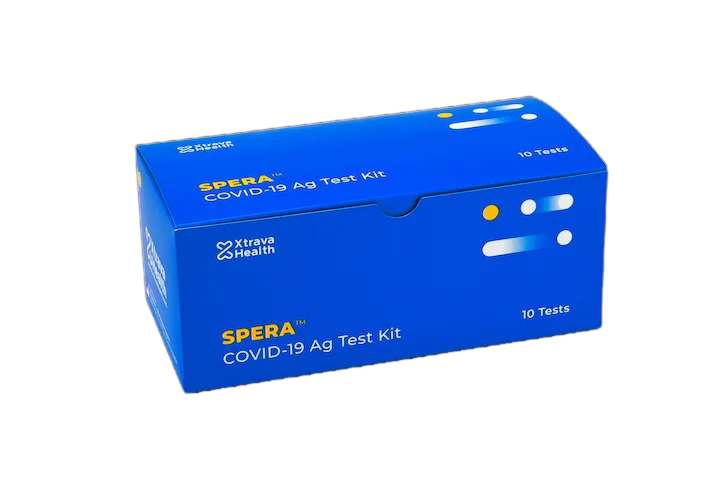
Spera™ Rapid Antigen Test(Box of 10)
Fast
Results in 10-15 minutes so your patients don’t have to wait.

Accurate
Developed in partnership with the NIH and manufactured in Japan, Spera™ is the most sensitive and highest quality rapid test on the market. Give your patients what they paid for.
Comfortable
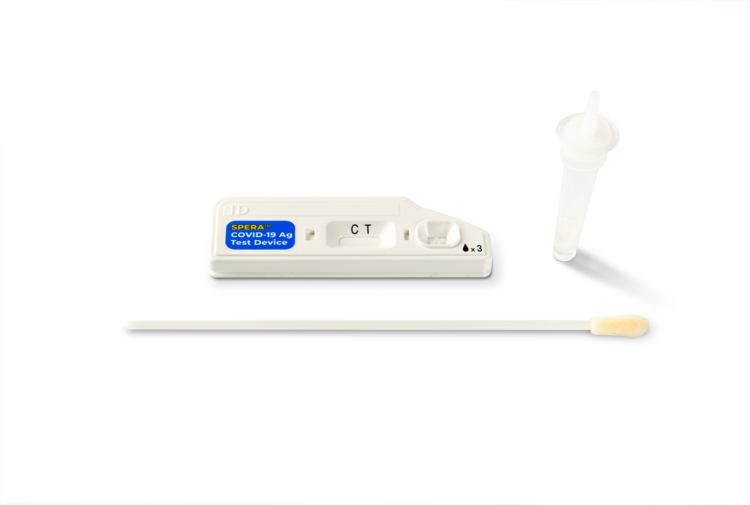
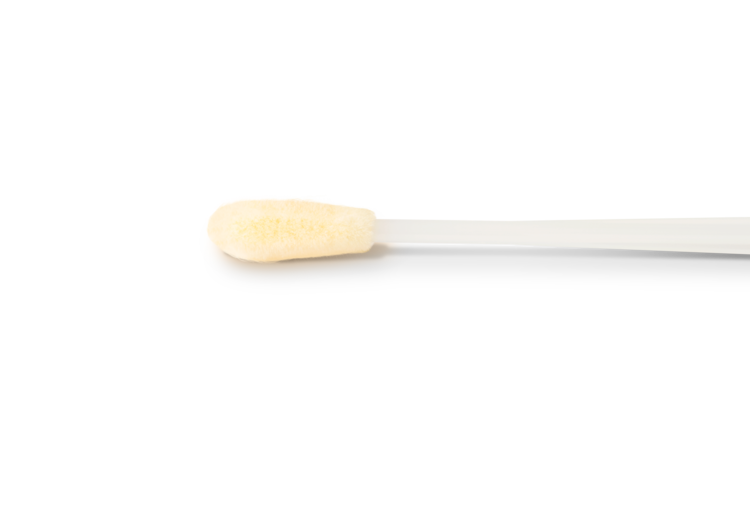
Lower nasal sample collection with state-of-the-art Copan flocked swabs for terrific patient comfort.
Convenient
Packaged in boxes of 10 so you can easily inventory, store, and apportion tests within your organization.
COVID-19 Rapid Test
Spera™ Rapid Antigen Test
The most sensitive rapid antigen test for detecting the 25+ strains of the new Omicron variant.
General Details
-
-
- Sensitivity: 92% for all variants. But ask us for the new Omicron data, even better.
- Specificity: 97% for all strains.
- Test Type: Rapid Antigen. Lateral flow immunoassay intended for the qualitative detection of SARS-CoV-2 nucleocapsid protein.
- Sample Type: Lower nasal swab
- Interfering Substances: No cross-reaction with species tested.
- Authorization: FDA EUA. CLIA certification required.
- Manufacturer: Xtrava Health (USA). Place of Manufacture: Japan
- Controls: Positive and negative controls available
- Shelf-life: 12 months
- Storage: 3–30°C
-
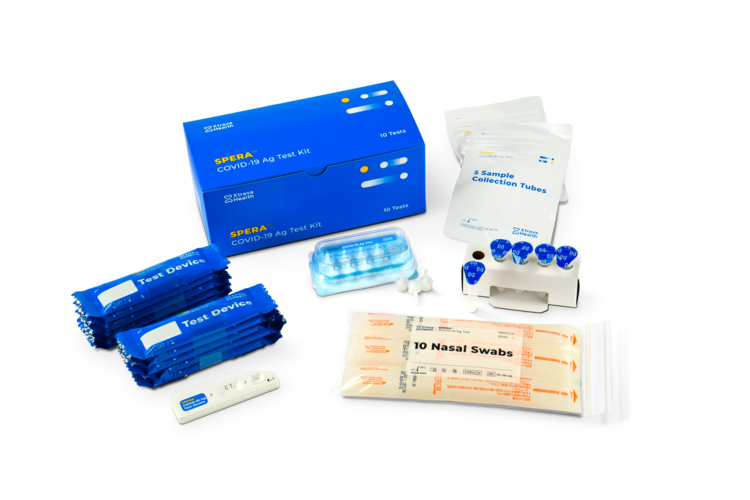
Box components
-
-
-
10 COVID-19 Test Cartridges
-
10 Sterile Shallow Nasal Swabs
-
10 Sample Collection Tubes with buffer
-
10 Sample Collection Caps
-
Convenient Sample Collection Tube Stand
-
Instructions for Use (IFU)
-
Quick Reference Guide
-
-
Spera™ Rapid Antigen Test
Manuals + Instructions
Intended Use
Qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal swabs from individuals suspected of COVID-19 by their healthcare provider within the first five (5) days of symptom onset. Emergency use of this test is limited to authorized laboratories.
In the USA, the SPERA COVID-19 Ag Test has not been FDA cleared or approved; but has been authorized by FDA under an EUA for use by authorized laboratories; use by laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C §263a, that meet the requirements to perform moderate, high, or waived complexity tests. The SPERA™ COVID-19 Ag Test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
CONTACT
Direct Connect
Phone
(310) 957-9891
