FLU A/FLU B RAPID TESTS
Status™ Flu A&B(Box of 25)
FLU A/FLU B RAPID TESTS
Status™ Flu A&B
An in vitro rapid qualitative test that detects influenza type A and type B antigens directly from nasal swab, nasopharyngeal swab and nasal aspirate/wash specimens.
General Details
-
-
- Meets the new FDA reclassification performance criteria
- CLIA Waived for swab specimen
- Includes Positive and Negative control swabs
- Innovative flip design with onboard sample extraction
- Differentiates between Influenza A and B
- Pre-measured developer solution capsule for increased accuracy and ease of use
- Flocked nasal swabs for improved patient comfort and superior specimen collection
-
Clinical Performance
-
-
-
PPA Influenza A: 89.2% (95% CI: 83.0-93.4%)
-
PPA Influenza B: 86.4% (95% CI: 80.1-90.9%)
-
NPA Influenza A: 99.4% (95% CI: 97.7-99.8%)
-
NPA Influenza B: 99.0% (95% CI: 97.1-99.7%)
-
-
Box components
-
-
- Status Flu A & B (25 tests)
- Nasal Aspirate Kit (25 tests)
-
FLU A/FLU B RAPID TESTS
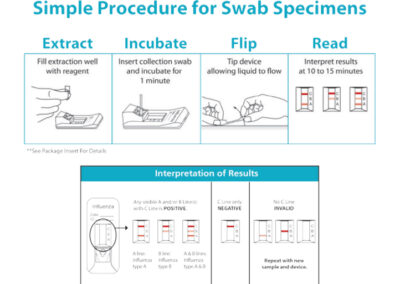
Manuals + Instructions
Storage and Handling
Test kits should be stored at 35-86°F. Reagents and materials are stable until the expiration date printed on the outer packaging. Do not use beyond the expiration date. The test device must remain in the sealed pouch until use. Do not freeze any contents of the kit.
Intended Use
Lateral flow immunoassay intended for the qualitative detection of SARS-CoV-2 nucleocapsid protein and Influenza A & B strains.
CONTACT
Direct Connect
Phone
(310) 957-9891