Monkeypox & Orthopox Virus Detection
U-TOP™ MPX Detection Kit(Box of 100 Reactions)
Monkeypox & Orthopox Virus Detection
U-TOP™ MPX Detection Kit
The First Monkeypox PCR Test
A molecular diagnostic kit for molecular detection of Monkeypox and Orthopox viruses in clinical samples using real-time PCR.
General Details
Detects:
-
-
- Monkeypox Virus
- Orthopox Virus Species
- Variola Virus (Smallpox)
- Vaccinia Virus
- Camelpox Virus
- Cowpox Virus
-
PCR Run-Time:
1 hour 15 minutes
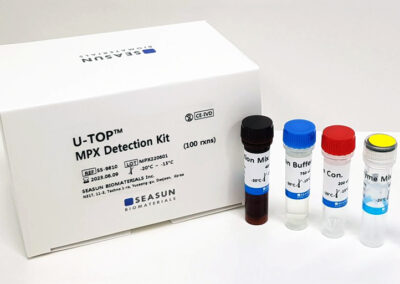
Box components
-
-
-
2X Reaction Buffer
-
Enzyme Mix
-
Reaction Mix
-
Positive Control
-
Negative Control
-
-
Specimen types
-
-
Follow WHO Recommendations
Febrile (Fever) Stage: NP or OP Swab
Lesion Stage: Lesion fluid, roof, crust
-
Monkeypox & Orthopox Virus Detection
Manuals + Instructions
Storage and handling
-
-
- Store reagents at -20° C. Once opened, use within 3 months.
- Store specimens at 2 – 8° C or -20° C within one hour after collection.
- Recommend immediate transport to lab.
- Store specimens at -70° C if not processed within 7 days
-
Intended Use
U-TOP™ MPX detection kit is a real-time PCR test intended for qualitative detection and discrimination of nucleic acid from the Monkeypox virus (MPXV) and other human-infective Orthopoxvirus (OPXV) species including Variola virus (smallpox), Vaccinia virus, Camelpox virus, Cowpox virus.
The kit targets one (1) specific region located on MPXV genome in FAM and one (1) generic region which is highly conserved among the human-infective OPXV genomes in HEX fluorescence channels. Primer and probe set targeting Human RNase P is used for internal/extraction control additionally in Cy5 fluorescence channel.
Positive results are indicative of the presence of target viral genome; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses.
Negative results do not preclude the viral infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
This test is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro diagnostic procedures.
Sample collection and transport should be followed the guidance released by the WHO.
CONTACT
Direct Connect
Phone
(310) 957-9891