IMMUNOASSAY RSV TEST
Quidel QuickVue® RSV Test(Box of 20)
IMMUNOASSAY RSV TEST
Quidel QuickVue® RSV Test
The QuickVue RSV Test is a dipstick immunoassay that detects respiratory syncytial virus (RSV) antigen (viral fusion protein) directly from nasopharyngeal swab, nasopharyngeal aspirate, or nasal/nasopharyngeal wash specimens for symptomatic pediatric patients (eighteen years of age and younger).
General Details
-
-
- Dipstick: Easy to use, approx. 30 seconds to 1 minute hands-on time
- Results in 15 minutes: Test and treat in the same office visit
- One reagent: Fewer steps, easy to perform, requires minimal training
- Two-color result: Easy to read and interpret
- 3 step procedure: Easy to use, fewer procedural errors
- Quick Reference Instructions: Clear and simple illustrations to guide the RSV test procedure and interpretation of result
- All components included in kit: Ready to use, no need for additional equipment
- Internal controls included: Provides verification of test strip functional integrity, increasing confidence in results
- External controls included: Facilitates internal laboratory quality control
- Room temperature storage: No refrigerator space needed. No need to wait for reagents to warm up. Rapid RSV tests can be run immediately as needed
-
Quidel QuickVue® RSV Test
Manuals + Instructions
Intended Use
The QuickVue RSV Test is a dipstick immunoassay which allows for the rapid, qualitative detection of respiratory syncytial virus (RSV) antigen (viral fusion protein) directly from nasopharyngeal swab, nasopharyngeal aspirate, or nasal/nasopharyngeal wash specimens for symptomatic pediatric patients (eighteen years of age and younger).
The test is intended for use as an aid in the diagnosis of acute respiratory syncytial viral infections. It is recommended that negative test results be confirmed by cell culture.
Negative results do not preclude RSV infection and it is recommended that they not be used as the sole basis for treatment or other management decisions. The test is intended for professional and laboratory use.
Clinical Details
-
-
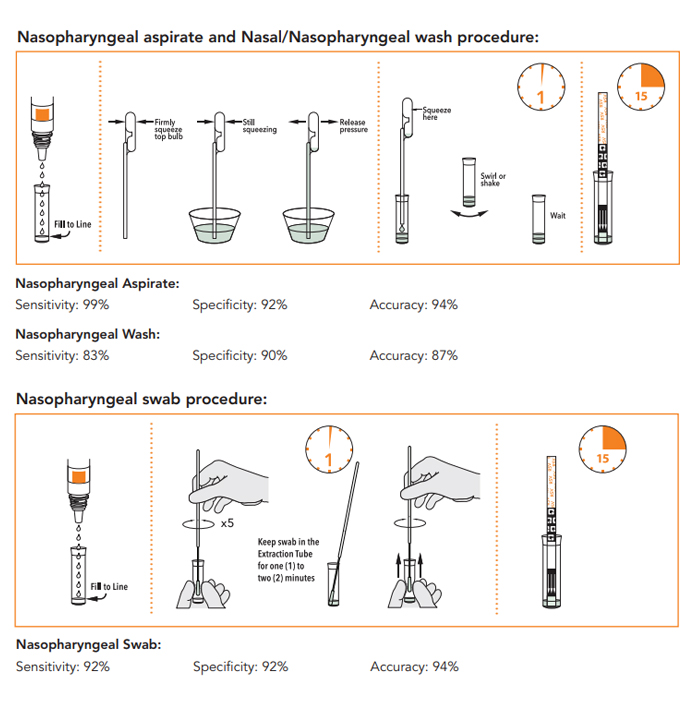
- Nasopharyngeal Aspirate:
Sensitivity: 99%, Specificity: 92%, Accuracy: 94% - Nasopharyngeal Wash:
Sensitivity: 83%, Specificity: 90%, Accuracy: 87% - Nasopharyngeal Swab:
Sensitivity: 92%, Specificity: 92%, Accuracy: 94%
- Nasopharyngeal Aspirate:
-
Storage
Store the kit at room temperature, 15°C to 30°C, out of direct sunlight. Kit contents are stable until the expiration date printed on the outer box. Do not freeze.
CONTACT
Direct Connect
Phone
(310) 957-9891