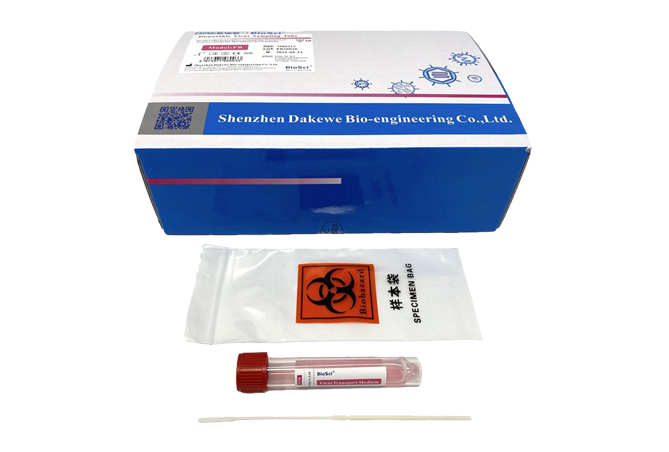
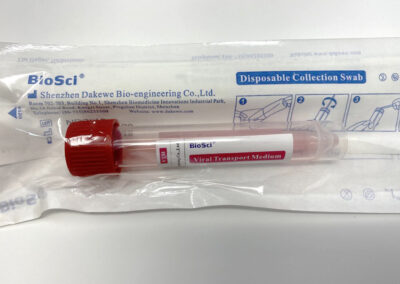
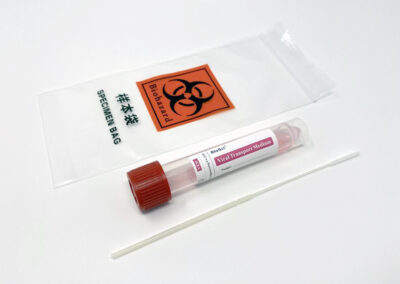
VIRAL TRANSPORT MEDIUM + SWAB KIT
BioSci® Collection KitsViral TRANSPORT MEDIUM + Swab KIT
BioSci® VTM Tubes
BioSci VTM uses the CDC standard formulation for VTM.
Authorized for distribution under FDA Enforcement Policy for Viral Transport Media. 510k pending.
Specifications
-
-
-
Intended Use: For the collection, preservation, and transport of clinical specimens to a testing laboratory.
-
Tube Type: 10mL polypropylene plastic tube with screw cap
-
Swab Type: Choice of nasopharyngeal or oropharyngeal/anterior nasal nylon flocked swabs.
-
Media Type: Sterile VTM, standard CDC formulation. (2mL)
-
pH: 7.1-7.5
-
Storage: Cold or room temperature, 2-25°C
-
Sterile: Yes.
-
Swabs: Gamma sterilized
-
Media: Filter sterilized
-
-
Media Composition
-
- Hank’s Balanced Salts Solution (HBSS)
- Phenol Red
- Bovine Serum Albumin (BSA)
- HEPES Buffer
- Gentamicin Sulfate
- Vancomycin
- Amphotericin B
Stability
-
-
-
Unused media may be transported and stored at room temperature, 15°C – 25°C.
-
Shelf life of unused media is 18 months.
-
After collection, samples should be stored and transported at 2 to 4°C for up to 48 hours.
-
If samples must be stored longer than 48 hours, it is recommended to cryopreserve them at -70°C.
-
-
Box Components
-
-
-
VTM tube, 2.0mL fill (20)
-
Sterile nasopharyngeal or oropharyngeal swabs (20)
-
VTM tubes and swabs are individually sealed together in a seal pouch.
-
3” x 7” biohazard collection bags (20)
-
Instructions for Use (1)
-
-
BioSci® Collection Kits
Manuals + Instructions
CONTACT
Direct Connect
Phone
(310) 957-9891